How cells prevent the translation of faulty mRNAs
October 2019. Scientists from Frankfurt and Mainz identify the RNA-binding E3 ubiquitin ligase Makorin Ring Finger Protein 1 (MKRN1) as a novel player in ribosome-associated quality control (RQC) against poly(A) translation.
Human cells maintain elaborate quality control mechanisms to detect and degrade aberrant mRNAs and proteins. Faulty mRNAs commonly arise from premature polyadenylation, which can disrupt the open reading frame and result in non-functional protein products. The RQC machinery detects ribosomes that have been stalled when running into poly(A) sequences and promotes their release and recycling. However, whereas peptide release and ribosome recycling by the RQC complex have been studied, less is known about the mechanisms that promote poly(A) recognition and initial ribosome stalling.
Researchers from BMLS at Goethe University Frankfurt and IMB in Mainz now identified the RNA-binding E3 ubiquitin ligase MKRN1 as a novel sensor of poly(A) sequences in RQC. In their study published in Genome Biology this week, they show how its unique domain architecture enables MKRN1 to bridge between specific RNA recognition and ubiquitin-mediated protein quality control. Using a multi-omics approach, they find that MKRN1 specifically binds at poly(A) sequences, interacts with poly(A)-binding proteins and translational regulators, and directly ubiquitylates ribosomal proteins. Reporter assays directly demonstrate that ribosomes no longer stall at poly(A) sequences when MKRN1 is depleted from the cells. These results cumulate in a functional model that MKRN1 acts as a roadblock on poly(A) sequences that prevents ribosomes from translating by ubiquitylating ribosomal proteins. The findings explain how cells are able to recognise the faulty mRNAs and prevents the production of harmful, non-functional proteins. This quality control is critical to maintain protein homeostasis and allows cells to continue to function despite making occasional mistakes in mRNA synthesis.
Full publication
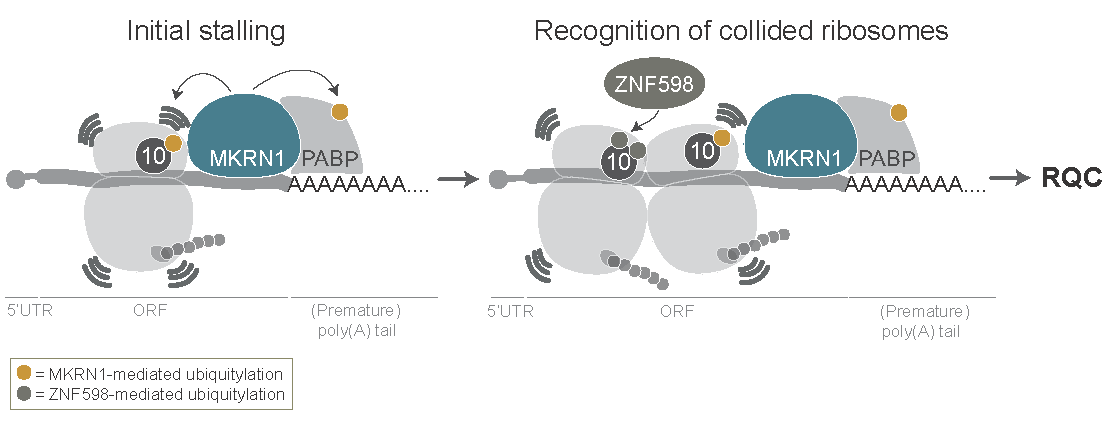
In the model of MKRN1 function, MKRN1 binds upstream of (premature) poly(A) tails via interaction with the poly(A)-binding protein. Ribosomes translating the open reading frame run into MKRN1 that acts as a roadblock to prohibit poly(A) translation. Upon contact with the translating ribosome, MKRN1 ubiquitylates and thereby stalls the ribosome. Trailing ribosomes collide and trigger ribosome-associated quality control.
Figure taken from Hildebrandt et al., Genome Biology
Contact:
Dr. Kathi Zarnack
Buchmann Institute for Molecular Life Sciences, Goethe University Frankfurt
kathi(at)bmls.de
Dr. Julian König
Institute of Molecular Biology (IMB), Mainz
j.koenig(at)imb-mainz.de
Dr. Petra Beli
Institute of Molecular Biology (IMB), Mainz
p.beli(at)imb-mainz.de
Publication:
Hildebrandt A, Brüggemann M, Rücklé C, Boerner S, Heidelberger JB, Busch A, Hänel H, Voigt A, Möckel MM, Ebersberger S, Scholz A, Dold A, Schmid T, Ebersberger I, Roignant JY, Zarnack K*, König J*, Beli P* (2019) The RNA-binding ubiquitin ligase MKRN1 functions in ribosome-associated quality control of poly(A) translation. Genome Biol 20, 216.
doi: https://doi.org/10.1186/s13059-019-1814-0
(* Corresponding authors)