Applications of heterobifunctional chemicals in cell biology
28 May 2021. It has been estimated that only 10% of our therapeutic proteome is currently druggable because classic chemical inhibitors require a well-defined hydrophobic binding site on the surface of the target protein, like growth factor receptors and protein kinases. The identification of thalidomide as a molecular glue opened a new era in drug development for degrading transcription factors and other tumorigenesis-associated proteins. This breakthrough also boosts the development of proteolysis-targeting chimeras (PROTAC). PROTAC is a heterobifunctional small molecule, consisting of a recruiter of an E3 ubiquitin ligase and a ligand of a protein of interest (POI) connected with a linker. In the presence of a PROTAC, POI will be catalytically degraded due to a chemically induced E3 ligase-POI interaction. Chemical inhibitors are generally used to prepare PROTACs, which, however, limits the application of the PROTAC technology to target POIs if no relevant chemical inhibitor is available.
In cooperation with the Zhou lab at DKFZ in Heidelberg and the Münch lab at Goethe University Hospital Frankfurt, Xinlai Cheng’s team at BMLS identified a new splicing factor SF3B1 activator from their previous high throughput screening called OCT4 inducing compound 2 (O4I2) because of its ability to support OCT4 activity in pluripotent stem cells. They utilized PROTAC technology and successfully converted O4I2 to a highly specific SF3B1 degrader. They demonstrated that the resulting PROTAC-O4I2 equally degraded SF3B1WT and SF3B1K700E mutation in cells and significantly increased survival in a Drosophila intestinal tumor model, implicating the application of noninhibitory chemicals in PROTACs. The result has been published in the journal Cell Chemical Biology (Link).

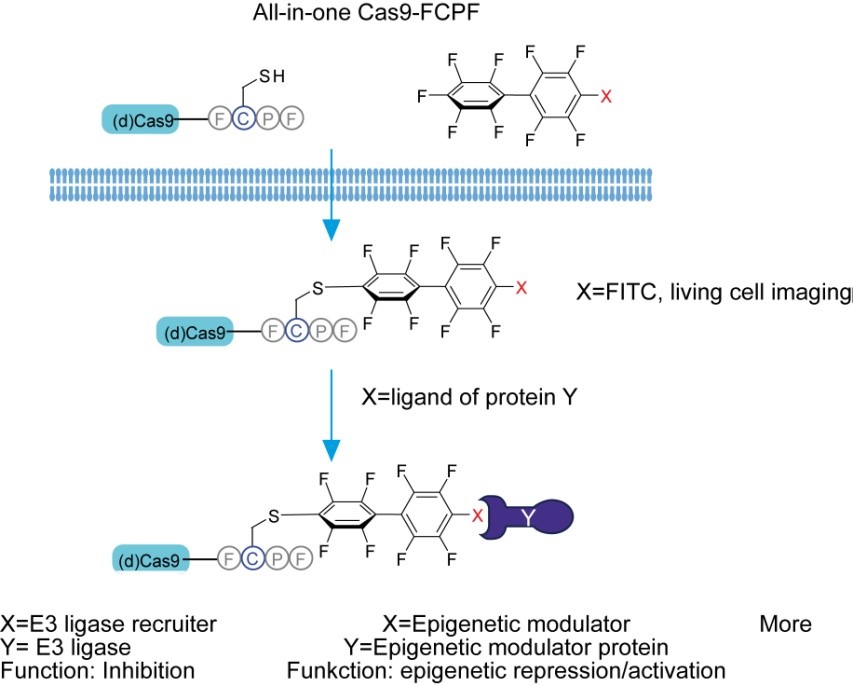
In order to expand the list of PROTACable proteins, Xinlai Cheng’s group in cooperation with the Münch lab and Stefan Knapp’s group at BMLS further achieved the degradation of exogenously expressed proteins by tagging Phe-Cys-Pro-Phe (FCPF) amino acid sequence in the presence of lenalidomid -functionalized perfluoroaromatics. In their recent publication in the journal JACS Au (Link), they report that this FCPF tagging system could be applied not only for degradation, but also for labeling proteins in live cells using fluorophore-conjugated perfluoroaromatics, demonstrating a useful chemical toolbox for the CRISPR/(d)Cas system.
Contact:
Xinlai Cheng, Buchmann Institute for Molecular Life Sciences and Institute for Pharmaceutical Chemistry, Goethe University Frankfurt, Frankfurt/Main, cheng@pharmchem.uni-frankfurt.de
Publications:
Gama-Brambila RA, Chen J, Zhou J, Tascher G, Münch C, Cheng X* (2021) A PROTAC targets splicing factor 3B1. Cell Chemical Biology, published online 27 May 2021. Link
Gama-Brambila RA, Chen J, Dabiri Y, Tascher G, Němec V, Münch C, Song G, Knapp S, Cheng X* (2021) A chemical toolbox for labeling and degrading engineered Cas proteins. JACS Au, published online 12 May 2021. Link